Whether you use that oatmeal jelly between your ears as a fine-tuned instrument of inquiry or as storage space for Monty Python quotes, you probably know that it works best when amply supplied with oxygen-rich blood. Your body, after all, is built from the cells upward for breathing and burning oxygen, and that goes double for neurons [source: Meek]. When this essential supply is cut off, a cascade of events affecting everything from your tissues to your cells is set in motion, the consequences of which can range from disability to death.
Over the past 70 years, medical research has revealed how refrigeration and resuscitation can go hand in hand, how otherwise life-threatening cold can be used to save lives and to delay this cycle of destruction.
Advertisement
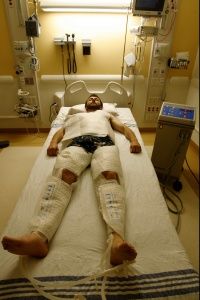
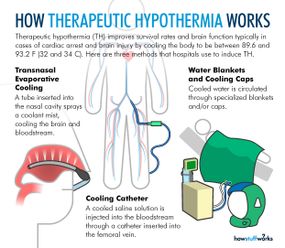
During therapeutic hypothermia (TH), also known as medically induced hypothermia or targeted temperature management, doctors lower oxygen demand in the brain by reducing a patient's body temperature to moderate hypothermic levels, thereby lowering the likelihood of cellular damage [sources: Deckard and Ebright; Gibson and Andrews]. Every 1.8 degrees Fahrenheit (1 degree Celsius) of body temperature reduction translates to an estimated 6-10 percent drop in cerebral metabolism and oxygen demand [sources: Adler at al.; Deckard and Ebright].
Typical procedures entail lowering core temperature past low normothermia (96.8-98.6 F, or 36-37 C) to a target range of 89.6-93.2 F (32-34 C) [sources: Deckard and Ebright; Gibson and Andrews]. That might not sound terribly frigid, but we're talking about core temperature, where a few degrees are all that separate a slight chill from heart and nervous system problems, organ damage and possible death [source: Davis].
In other words, don't try this at home.
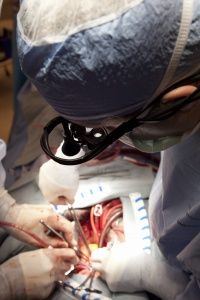
Through careful monitoring and temperature control, doctors can apply TH to cases that call for either intervention or prevention. Intervention involves curtailing the damage of a recent incident, one that deprived the brain of blood flow and oxygen. The two most common TH interventions involve cardiac arrest patients who don't wake up after circulation returns and newborns suffering from neonatal ischemic encephalopathy, an illness resulting from oxygen deprivation that can cause death, mental retardation, cerebral palsy and/or epilepsy, among other neurological effects [sources: Andrews; Deckard and Ebright; Lai and Yang; Leary; Merck Manual]. As a preventative measure, TH extends operating time and protects the brain during open heart surgery [source: Texas Heart Institute].
Over the past few decades, researchers have begun exploring using TH in treating specific types of stroke, heart attack, respiratory problems and injuries to the brain and spinal cord. Possible uses in treating acute kidney injury and managing cancer are in the early stages [sources: Encyclopedia Britannica; Leary].
Advertisement